Rutherford scattering
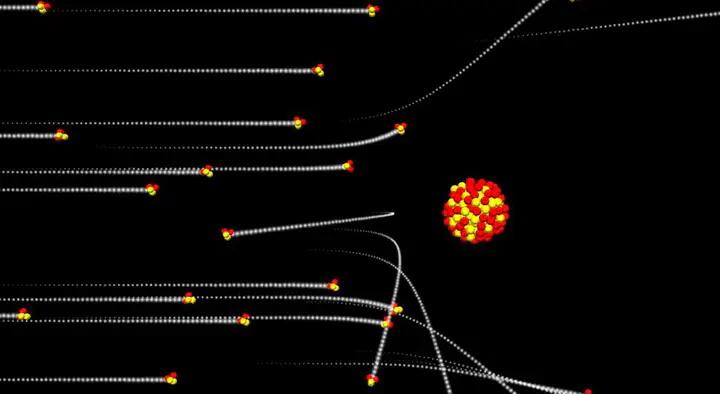
The purpose of this experiment is to calculate the atomic number of aluminum by knowing the depth of two different sheets and the densities of both materials then measuring the count rates of each at different angles, finally by knowing the atomic number of one of the materials we can calculate the atomic number of the other, this resulted in a calculated value for the atomic number of aluminum of 42 particles within the nucleus of an atom. Furthermore the experiment allows us to draw conclusions based on observations observed, these conclu- sions are directly responsible for the standard model of the atom and they took place as follows: • The atom contains a dense nucleus which is positively charged • The atom is mostly made of empty space • Electrons are no present in the nucleus but are in fact somewhere else. (opposing the plumb pudding model)